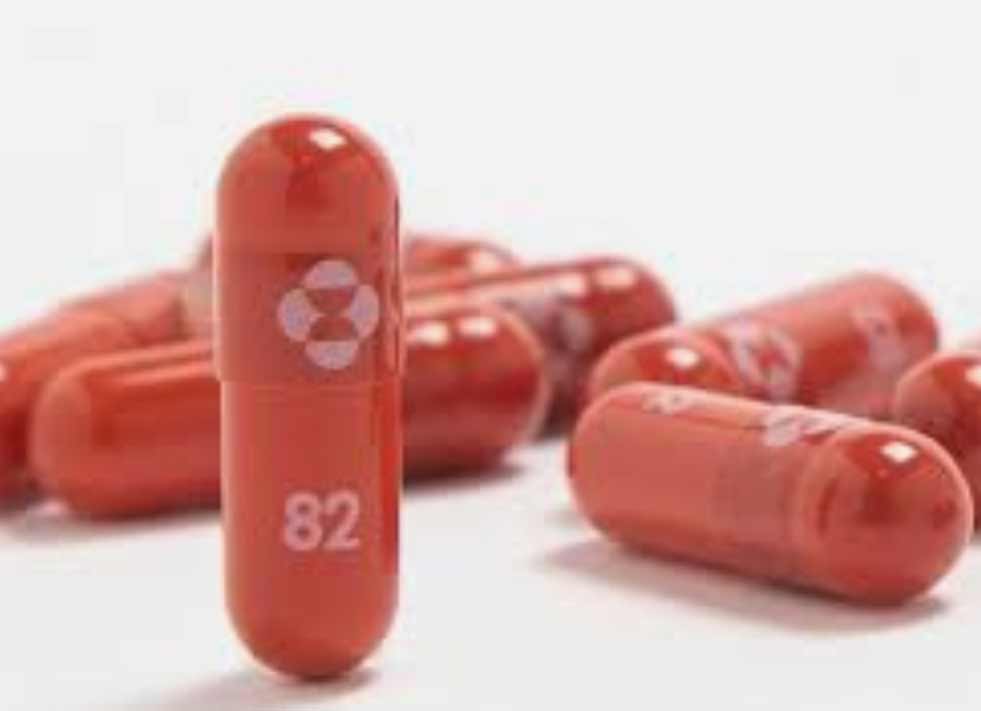
The Medicines and Healthcare products Regulatory Agency (MHRA) announced. Today that the antiviral Lagevrio (molnupiravir) is safe and effective. At reducing the risk of hospitalization and death in people with mild to moderate COVID-19. Who are at increased risk of developing severe disease.
This follows a rigorous review of its safety, quality, and effectiveness. By the UK regulator and the government’s independent expert scientific advisory body. The Commission on Human Medicines, making it. The first oral antiviral for the treatment of COVID-19 to be approved.
Health and Social Care Secretary Sajid Javid declared, “Today marks a historic day for our country, as the UK becomes the first country in the world to approve an antiviral that people can take at home for COVID-19.””This ground-breaking treatment will soon be a game-changer for the most vulnerable and the immunosuppressed, enabling them to receive it.”
Dr. June Raine, MHRA chief executive, said “following a rigorous review of the data. Our expert scientists and clinicians have determined that Lagevrio (molnupiravir) is safe and effective for those at risk of developing severe COVID-19 disease, and they have granted its approval.”
For official details on the first oral antiviral for Covid-19. Visit the government website. If you have any concerns and would like to speak with a doctor at your convenience and in your own home or place of work, why not book an appointment with us today?